Investigating Boyle's Law with PocketLab
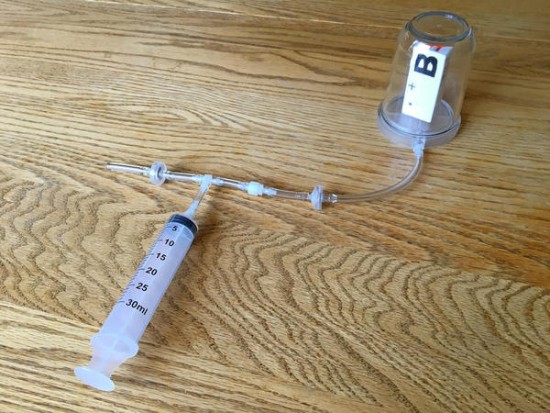
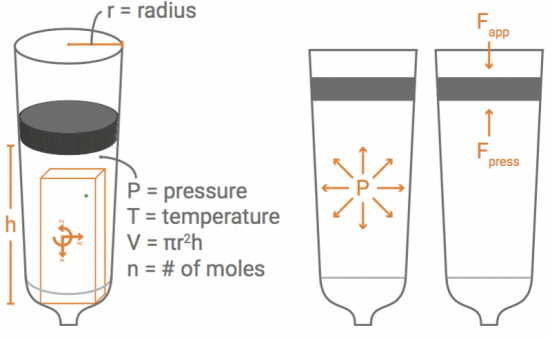
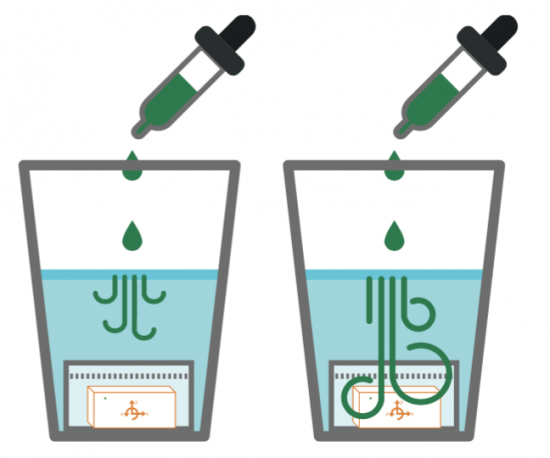
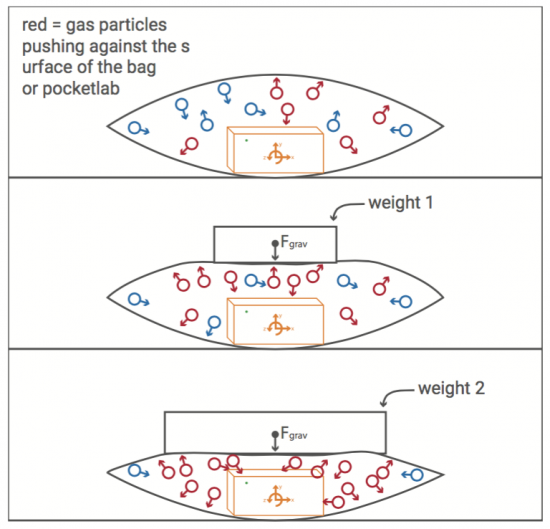
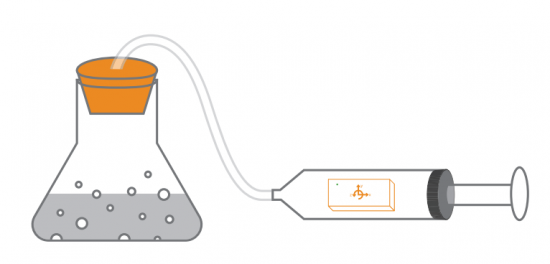
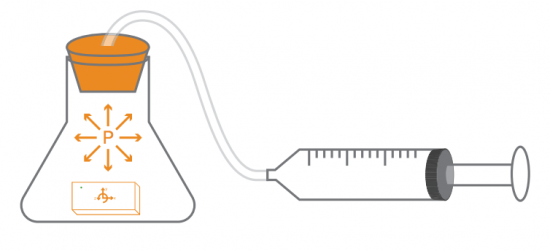
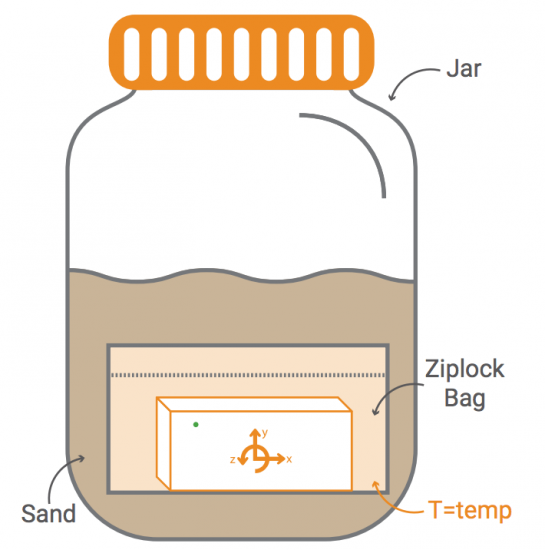
With a pressure sensor built into PocketLab, there must surely be some way to investigate Boyle's Law. This law states that pressure and volume of an ideal gas are inversely proportional to one another provided that the temperature and amount of gas are kept constant within a closed system. What is needed is a closed system that is large enough to hold PocketLab in a way that pressure can be sensed while changing the volume of the enclosed gas (in our case, air).