Gay-Lussac's Law states that when the volume of a container of gas is held constant, while the temperature of the gas is increased, then the pressure of the gas will also increase. In other words, pressure is directly proportional to the absolute temperature for a given mass of gas at constant volume. Although this is, strictly speaking, true only for an ideal gas, most gases that surround us behave much like an ideal gas. Even ordinary air, which is a mixture of gases, can behave like an ideal gas.
In this experiment, a PocketLab that is sealed inside a Mason jar can be used to verify Gay-Lussac's Law as well as extrapolate a value for the absolute zero of temperature. The PocketLab is set to "Two-Graph" mode, recording pressure in mBar and temperature in celsius degrees. Considering the PocketLab specifications for the temperature sensor, it is seen that the allowed range is from -20C to 85C. It would be perfect if we could measure the pressure of the air in the Mason jar for three different temperatures covering the allowed range. The photos in the figure below show three such possibilities.

The photo on the left shows PocketLab sealed in a Mason jar on a table at room temperature, about the middle of the allowed range. The photo in the middle shows the PocketLab Mason jar in a freezer, which will give us a temperature near the low end of the allowed range. The photo on the right shows the PocketLab Mason jar in an oven set to a maximum temperature of 170F (77C), just a little below the high end of the allowed range. It took about an hour for the PocketLab temperature sensor to reach the desired values in the freezer and in the oven, so patience is required.
For safety, protective goggles should be worn. In addition, gloves should be worn when removing the jar from the freezer, as it is cold enough to cost frost bite if handled too long. Gloves should also be worn when removing the jar from the oven, as it will be at a temperature that is not too far from that of boiling water. It is also essential to monitor the temperature on the iPhone to make sure that it doesn't exceed the high end of the allowed range. It should be removed from the oven and the oven turned off a little before reaching the high end of the allowed range. For the author, the stainless steel freezer and oven did not stop PocketLab from communicating data with the iPhone setting on a nearby counter. The author also kept the iPhone charging cord attached during the experiment to avoid running out of charge on the iPhone battery.
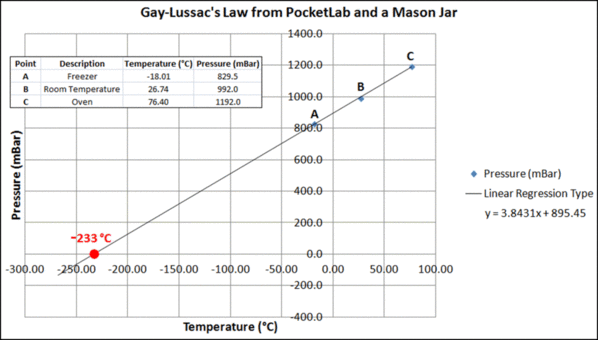
The Excel graph shown below summarizes the experimental results. The three data points fall very close to a straight line obtained by doing a linear trend/regression. The line is extended to the left until it reaches the temperature axis. At that temperature, -233C, the pressure would be zero. The value -233C can be obtained from the regression equation by setting y to 0 and solving for x. With the absolute zero of temperature at -273.15C by international agreement, our value of -233C represents an error of about 14.7%. It would likely promote a good classroom conversation to discuss possible causes for this error.