Exploration
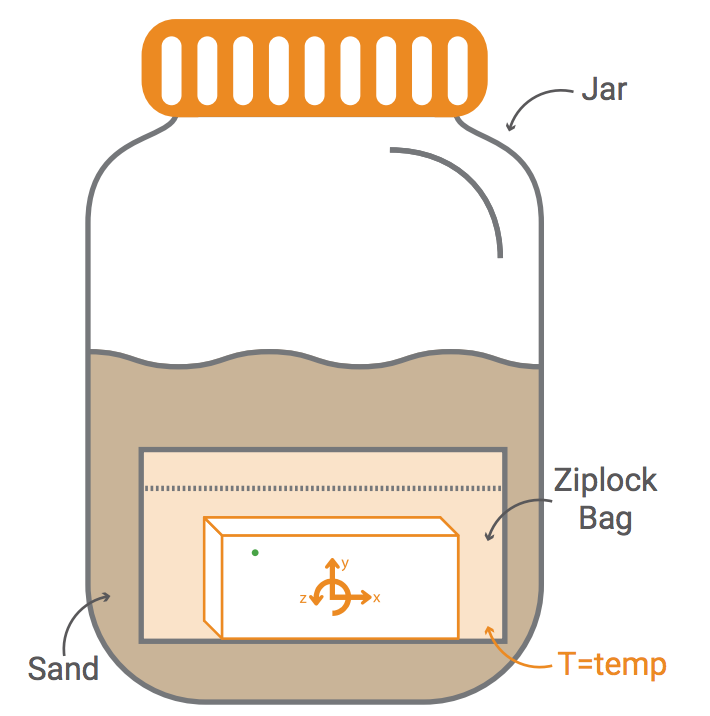
The law of conservation of energy states that the total energy of an isolated system remains constant. Over time, all energy is conserved. It is neither created nor destroyed-instead it transfers from one form to another. When shaking a jar of sand, what happens to the temperature of the sand? Explore how this relates to the law of conservation of energy.
Objective
In this experiment, students will:
1) Observe how kinetic energy transfers to thermal energy.
2) Use observations of heat transfer to better understand the law of conservation of energy.
3) Trace the thermal energy in the sand to energy from sunlight.
Download PDF for complete lab activity
Subject
Grade Level

