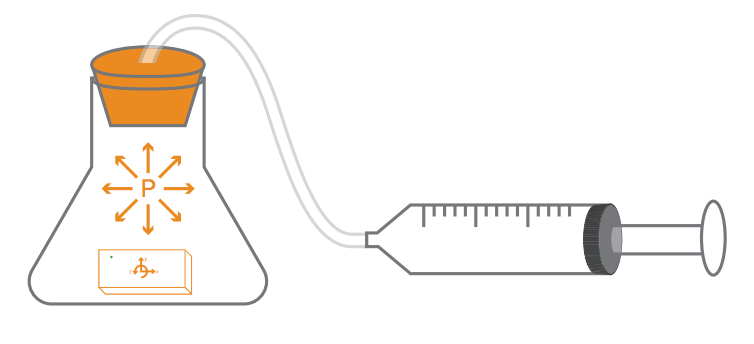
Exploration
Explore air pressure, temperature, and volume and how they work together. In a syringe sealed to an Erlenmeyer flask , when the syringe’s plunger moves back and forth, the volume of air in the syringe and f ask changes. Will the pressure also change if the temperature of the air sealed in the syringe and f ask changes? A PocketLab can be placed inside the Erlenmeyer f ask to measure the change in pressure as the the volume and temperature change.
Objective
In this experiment, students will:
1. Develop a model that describes the relationship between air pressure, volume, and temperature.
2. Describe what is happening to the air particles when there is a greater or less air pressure.
3. Describe what is happening to the air particles when there are changes in temperature and how that relates to the measurement of temperature itself.
Download PDF for complete lab activity