What is Ocean Acidification? (HS-Secondary)
Climate change is at the forefront of environmental concerns and it often revolves around atmospheric carbon dioxide concentration and its effect on average surface temperature. However, carbon dioxide concentration is not only rising in the atmosphere but in the ocean as well. The source of this dissolved carbon dioxide is the rising atmospheric carbon dioxide levels we hear so much about, as carbon dioxide is soluble in water. Of the 37 Gt of carbon dioxide we release globally each year, 9.25 Gt ends up dissolved in the ocean—which is a whopping 25%. While this has led to a decrease in the severity of changes to the atmosphere, it has also led to another problem—that of ocean acidification. Carbon dioxide, when dissolved in water, reacts to form carbonic acid, which is causing widespread acidification in the top layer of our oceans.
If you're teaching in K-MS check out this simplified version of the experiment
Ocean and Atmosphere in a Box
Many experiments investigate the process of acidifying water by the addition of carbon dioxide or via a proxy acid, such as vinegar (acetic acid). However, this experiment specifically demonstrates the equilibrium process through which atmospheric carbon dioxide enters the ocean and decreases pH.
Air to Water
This experiment requires a significant amount of time to reach the equilibrium necessary to see a notable color change in the pH indicator. However, it demonstrates that when carbon dioxide increases in the air, it will be absorbed by water, which will then—through various equilibrium processes—cause ocean acidification.
The carbon dioxide is produced via Alka-Seltzer tablets in a minimal amount of water—causing a drastic increase in concentration in the air. The carbon dioxide in the air will slowly dissolve in the water and change the pH of the water sample. Note that this change is only noticeable after a couple of hours but will intensify over half a day or more.
Materials
- PocketLab Air
- Sealable container (suggest sterilite gasket boxes as they are airtight)
- Tap water or distilled water
- 400 ml beaker or translucent container
- 100 ml beaker or translucent container
- Bromothymol blue or Bogens universal indicator
- 2 Alka-Seltzer tablets
Experiment
- Fill a large beaker or translucent container with 200-300 ml of water
- Add a couple drops of Bogens universal indicator or bromothymol blue until a strong color is observed; note the color and then chill in fridge until the solution is cold throughout
- Place the chilled beaker inside a large sealable container
- Fill your small beaker/container with water and then place this beside your larger beaker inside the sealable container
- The diameter of beaker/container and volume of water should be just enough to fit 2 Alka-Seltzer tablets
- A taller container is best for this to ensure the bubbles do not spill out into the larger container
- Setup and add your PocketLab Air to the container and start recording
- Quickly add 2 Alka-Seltzer tablets to the small beaker/container and then seal the large container immediately
- There should be an immediate spike in carbon dioxide which will likely max out the carbon dioxide sensor at 5000 ppm and over time the indicator color should slowly change
- Change is noticeable after ~2 hours, but giving a day to allow equilibrium to be reached will lead to a more noticeable change in color
Water to Air
This second experiment is commonly used for hands-on ocean acidification experiments and is faster than the first experiment. However, it is less representative of the real-world phenomena. This time, the experiment is going backward. Carbon dioxide is being both produced and captured by the water, and the excess is entering the air.
Materials
- PocketLab Air
- Sealable container (suggest sterilite gasket boxes as they are airtight)
- Tap water or distilled water
- 400 ml beaker or translucent container
- 100 ml beaker or translucent container
- Bromothymol blue or Bogens universal indicator
- Alka-Seltzer tablet
Experiment
- Fill a large beaker or translucent container with 200-300 mL of water
- Add a couple drops of Bogens universal indicator or bromothymol blue until a strong color is observed; note this color
- Place this beaker/container inside a large sealable container
- Setup and add your PocketLab Air to the container and start recording
- Quickly add an Alka-Seltzer tablet to the beaker/container and then seal the large container immediately
- The indicator should quickly show that the water is acidic, and following soon after, there should be a sharp spike in carbon dioxide within the air of the container which depending on container volume may max out the carbon dioxide sensor at 5000 ppm
Results
|
Indicator |
Type of water |
Initial color |
Change in color |
|
Bogens universal indicator |
Tap water |
green to yellow |
yellow to orange-red |
|
Distilled water |
bright yellow |
deeper yellow to orange-red |
|
|
Bromothymol blue |
Tap water |
light blue |
light yellow |
|
Distilled water |
light yellow |
lighter yellow to almost colorless |
|
|
|
|
Bogens universal indicator in tap water before (right) and after (left) experiment |
Bogens universal indicator in distilled water before (right) and after (left) experiment |
|
|
|
|
Bromothymol blue in tap water before (right) and after (left) experiment |
Bromothymol blue in distilled water before (right) and after (left) experiment |
The most noticeable indicator change occurs when using tap water. However, distilled water is useful to prove to students that the change in indicator color is from the reaction of water with the Alka-Seltzer tablet rather than contaminants in the water. Note that when demonstrating color change to students, it is best when they can compare with a sample of tap or distilled water with indicator in it. Having these controls samples prepared at the same time as the sample clearly shows that the change in indicator color happens due to conditions within the box rather than just over time in open air.
Introductory Background
The oceans have changed from a pH of 8.16 before the industrial revolution (1850) to 8.07 in 2014. While this change may seem minor, it is about a quarter increase in the amount of acid; which is a drastic change in less than 200 years. This increase in acidity is due to increasing atmospheric carbon dioxide, which dissolves into the ocean—becoming carbonic acid. With the predicted increase in atmospheric carbon dioxide concentration, it is modeled that pH will drop to 7.96 if we follow the Paris agreement and 7.64 if we continue with our current emissions. That is a 190% and 230% increase in the amount of acid. Lots of common sea life have hard structures (skeletons or endoskeletons) made of limestone like coral, crab, lobster, mollusk, sea urchin, starfish, and sand dollars. All of these organisms require the oceans to be quite basic in order to form these structures. With our current emissions of atmospheric carbon dioxide, by 2095 all of the arctic and southern oceans and parts of the North Pacific Ocean will be acidic enough to make most of these organisms unable to form these structures. This will cause extreme disruptions in the oceans—especially when it comes to corals, which act as homes and the foundation for their entire ecosystem. Currently, this additional carbon dioxide is concentrated in the surface water, but between the next 300 to 1,000 years, this will start to be cycled throughout the deeper water of the oceans—affecting even more ocean communities. These disrupted ecosystems mean a loss of food, tourism, and ecosystem services for coastal communities globally.
For diagrams and photos continue reading in the expert background
Expert Background
Change to Date
To date, the ocean has dropped by 0.1 from its initial pH of 8.16, at the start of the industrial revolution, to 8.07. This may seem like a minor change, but a quick look at the formula for pH tells a different story.
Since the conversion of hydronium ion concentration to pH involves a logarithm, small pH changes are huge changes from the initial concentration. A difference of one in pH is a difference by a factor of ten in terms of the hydronium ion concentrations. The negative in the formula can also be confusing for students as it means that the decrease in pH means an increase in hydronium ion concentration. Note that although we are discussing the presence and further addition of acid to the oceans, our pH is still basic. This is due to ocean pH largely being controlled by borate ions (B(OH)4(aq)), which have remained unchanged, rather than carbonic acid. The current and predicted changes from the industrial revolution can be seen below.
Current and predicted to 2100 charges in pH and hydronium ion concentration
|
Year |
Global average pH |
Hydronium ion concentration (mol/L) |
|
Before industrial revolution (1850) |
8.16 |
6.9 E-9 |
|
Current (2014) |
8.07 |
8.5 E-9 |
|
1.1% decrease |
23% increase |
|
|
2100 Best possibility (Meet unofficial Paris agreement of 1.5oC) |
7.96 |
2.0 E-8 |
|
2.5% decrease |
189% increase |
|
|
2100 Business-as-Usual |
7.64 |
2.29 E-8 |
|
6.4% decrease |
232% increase |
Percent change in global average pH and hydronium ion concentration is relative to the 1850 value. 2100 values were found by using IPCC predicted values for atmospheric carbon dioxide concentration and models for ocean acidification developed by reference 3.
Why is it becoming acidic?
Why does this increase of carbon dioxide cause the entire ocean to acidify? The answer lies in the chemistry of carbon dioxide. Once it is in aqueous form, it goes through a series of reactions outlined below.
Reactions of Carbonic Acid
- Formation of carbonic acid (H2CO3) via the reaction of atmospheric CO2 and water (eq 1-2)
*Note that carbonic acid is a diprotic acid meaning it can lose two hydrogens.
eq 1.
eq 2.
- Carbonic acid then reacts with water dissociating into bicarbonate (HCO3-) and hydronium ion (H3O+) (eq 3.).
eq 3.
- A small percentage of bicarbonate ions then react with water again dissociating into a carbonate (CO32-) and another hydronium ion (eq 4.).
eq 4.
The speciation diagram below indicates that almost all the carbon dioxide that dissolves in the ocean only dissociates once to bicarbonate. This means there is an overall increase in the amount of bicarbonate and hydronium ions when carbon dioxide dissolves in the ocean.
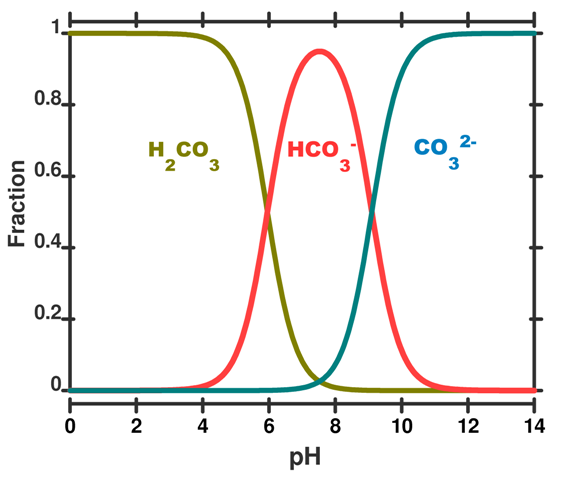
Solubility of Calcium Carbonate
- The major source of carbonate ions in the ocean is the dissolution of calcium carbonate (eq 5.)
(eq 5.)
Referring to the speciation curve above, at the current ocean pH carbonate would prefer to exist as bicarbonate which requires hydronium to react with (eq 6.)
- The current pH of the ocean as seen in the speciation curve above drives carbonate ions to form bicarbonate ions. This requires hydronium ions to react with which are available from the dissociation of carbonic acid. (eq 6.)
(eq 6.)
- The removal of carbonate ions means more calcium carbonate dissolves to reach equilibrium for carbonate ions (eq 5.)
Below is a well laid out diagram of the reactions outlined above. This diagram however, does not show carbonate coming from calcium carbonate explicitly but instead indicates deformed shells which are made of the ever increasingly dissolving calcium carbonate.
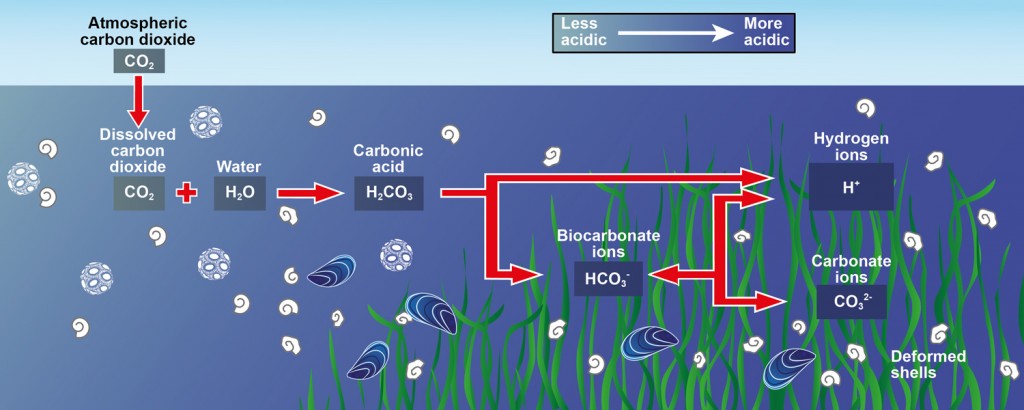
Effects of Ocean Acidification
Calcium carbonate is what makes up the skeletons of corals and the shells of almost any hard-bodied marine organism you can think of. For these organisms to form these structures the water must be supersaturated with calcium and carbonate so that calcium carbonate forms. These ions are sparse in the vast ocean, so the formation of these crystalline structures requires that organisms bring these molecules together, rather than random chance. Ocean acidification shifts the equilibrium so that calcium carbonate dissolves more easily, making it more energy-intensive for these organisms to form and maintain their hard structures. However, in the future, if the oceans become simply saturated, or worse, undersaturated with respect to calcium or carbonate, most organisms will be unable to form these structures at all. This makes it hard for these creatures to survive, having a detrimental impact on the ecosystems that they are a part of.

Keep in mind that the severity of this depends on the species due to differences in the structure of their calcium carbonate at the molecular level. The most soluble form of calcium carbonate is aragonite which makes up the shells of mollusks and skeletons of warm and cold-water corals. Calcite is a less soluble form that makes up the skeletons or shells of some phytoplankton, foraminifera (shelled single-cell protists), starfish, sand dollars, sea urchins, and crustaceans (crabs and lobsters). Predictions of which oceans will be unsaturated in terms of aragonite and calcite over the next century can be found below. This clearly demonstrates that marine organisms using aragonite will be under threat in a much shorter time frame.
Preidcted aragonite and calcite undersaturation under RCP8.56
|
Year |
Aragonite undersaturation |
Calcite undersaturation |
|
2020 |
Parts of the Arctic Ocean |
|
|
2050 |
All of the Arctic Ocean Parts of South Ocean |
|
|
2095 |
All of the South Ocean Parts of the North Pacific Ocean |
Parts of Arctic Ocean |
A large portion of the world’s population relies heavily on the ocean for their way of life and livelihoods. The loss of species that depend on calcium carbonate will have drastic effects across the ocean ecosystem and could lead to ecosystem collapse in many areas. This could remove ecosystem services and revenue that the ecosystem previously supplied to local populations. The loss of any species will disrupt the ecosystem, but the loss of keystone species upon which the ecosystem is built can be devastating. For example, warm and cold-water corals are not simply another species in the food web, but the basis of the entire ecosystem. This is because they form the ecosystem itself with all other organisms living in, on, and among them. Current predictions of the business-as-usual pathway (RCP8.5) show that 99% of coral will die by 2100 from a combination of factors. A significant factor being ocean acidification which will not only cause coral death but stop populations from recovering due to their inability to build a skeleton. Losing coral reefs will also mean a loss of their natural wave protection, food security, tourism, biological diversity, and natural beauty.
The Carbon Cycle
This might leave you wondering how the ocean has dealt with the natural sources of carbon dioxide prior to the start of the industrial revolution. While the ocean is a sink for atmospheric carbon dioxide, it does not remain there permanently. The full carbon cycle can be found in the image below. The process of carbon dioxide mixing throughout the ocean can take 300-1,000 years, and this must occur before it can enter sediment and be cycled back to the terrestrial systems. This means there are far reaches of the ocean that have not yet been affected by the increase in carbon dioxide, but also that much of the carbon dioxide being absorbed is staying near the surface, concentrating and causing more severe effects. Rather than cycling into deeper water, diluting the issue, our unrelenting carbon dioxide contributions will have severe effects on shallower water until the deeper water slowly becomes similarly impacted as it is cycled down in turnover. Carbon dioxide is less soluble at lower depths due to the high pressure which can lead to isolated upwelling into surface waters.
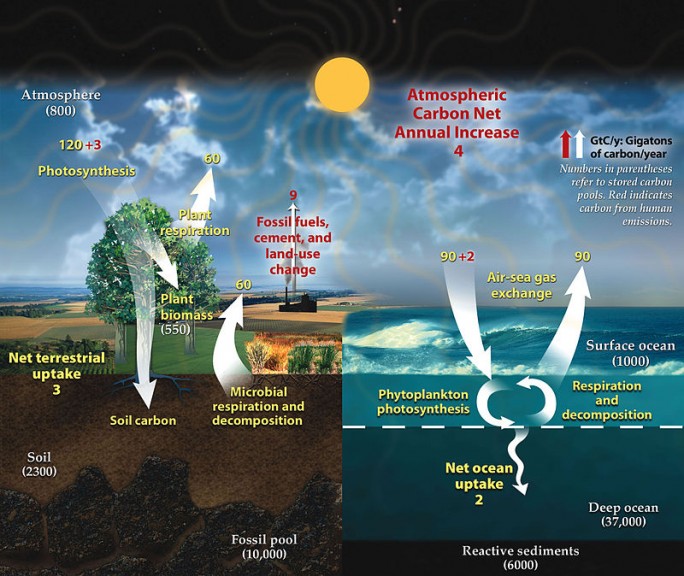
References
(1) Mackie, D. AO not Okay. https://skepticalscience.com/Mackie_OA_not_OK_post_1.html (accessed Aug 14, 2019).
(2) Wayne, G. P. The Beginner's Guide to Representative Concentration Pathways. https://skepticalscience.com/rcp.php?t=3#landuse (accessed Aug 20, 2019).
(3) Bozlee, B. J.; Janebo, M.; Jahn, G. A Simplified Model to Predict the Effect of Increasing Atmospheric CO2 on Carbonate Chemistry in the Ocean. J. Chem. Educ. 2008, 85, 213.
(4) AnonymousOcean Acidification. https://www.pml.ac.uk/Research/Research_topics/Facing_the_challenge_of_new_pollutants/Ocean_acidification?page=2 (accessed Aug 14, 2019).
(5) Liittschwager, D. Pteropod Dissolution. http://liittschwager.com/Pteropod_dissolution_stack_to_grid_of_9_larger_text-photo.html (accessed Aug 14, 2019).
(6) Feely, R. A.; Doney, S. C.; Cooley, S. R. Ocean Acidification: Present Conditions and Future Changes in a High-CO₂ World. Oceanography 2009, 36.
(7) Riebeek, H.; Simmon, R. The Carbon Cycle. https://earthobservatory.nasa.gov/features/CarbonCycle (accessed Aug 14, 2019).


