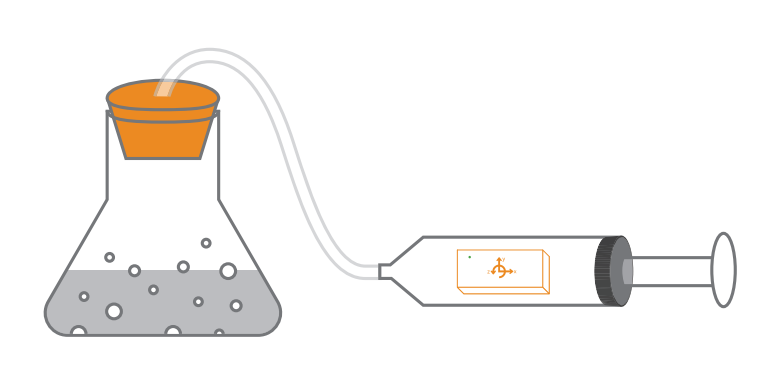
Measuring Pressure Change from Chemical Reaction
Exploration
After a change occurs, if the molecules of the chemicals involved do not change, it is only a physical change. Ice melting to water is an example of this. A change has occurred, but the H2 0 as ice, remains H2 0 as water. If however the molecules of the chemicals involved do change to form new chemicals, then a chemical change has occurred.
When the coal in a grill burns during a cookout, the carbon (C) from the coal combines with the surrounding oxygen (O2 ) to form a new gas, carbon dioxide (CO2 ). In this investigation you will use PocketLab to explore chemical reactions while measuring their intensity.
Objective
In this experiment, students will:
1. Examine data before and after a change has taken place to analyze and prove whether it was a chemical change.
2. Interpret the collected data to determine how the substances may have changed.
3. Determine whether there is a greater change if more of the substances are involved in the reaction.
Download PDF for lab activity